 Diffusion is where particles move from a high concentration to a low concentration. Think of it as stuff spreading out (like perfume being sprayed into the air)
Diffusion is where particles move from a high concentration to a low concentration. Think of it as stuff spreading out (like perfume being sprayed into the air)1st EXPERIMENT:
- Potassium Manganate(VII) and water, if you have a beaker of water, slowly place some potassium manganate(VII) to the bottom. The purple "stuff" will diffuse out into the water spreading a purple colour.
- Basically the potassium manganate(VII) particles are diffusing into and around the water. From 1.1, we know that liquid moves in a random motion which therefore causes the potassium manganate(VII) to spread out evenly.
- The experiment can then be repeated to test dilution. If you add water to the potassium manganate(VII) solution, the particles would spread out further due to the random motion evenly spreading it out. This results in the solution being less purple.
2nd EXPERIMENT:
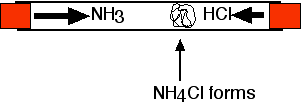
- Ammonia and Hydrogen Chloride, when the two are placed into a tube eventually a white ring of ammonium chloride forms. This is because the ammonia diffuses from one end and vice versa with the hydrogen chloride. From the picture we can see that the ring is not in the middle, this is due to the particles of ammonia being smaller and lighter hence being able to travel faster than hydrogen chloride.

3rd EXPERIMENT:
- Bromine Gas and Air, are a great to test for diffusion because of the strong brown colour (and smell). All you need is: two cups and a separator. If you fill one cup with bromine gas and the other with air and put them on top of each other, between a separator, then remove the separator you will see brown bromine gas diffuse into the air. As of the liquid, the random motion causes it all to spread out evenly.
No comments:
Post a Comment